Move faster with automated testing for Adobe Acrobat Sign
Home Applications Adobe Sign
• ENTERPRISE-GRADE E-SIGNATURES WITH COMPLIANCE AND AUTOMATION BUILT IN
Get Automated Testing, Validation Lifecycle Management, and Application Lifecycle Management in a Single App
Adobe Acrobat Sign’s enterprise-level security reduces your compliance risk—no matter your industry or where you do business. Configure Acrobat Sign Solutions to meet industry specific regulations around the world, including HIPAA, FERPA, GLBA, and FDA 21 CFR Part 11.
ProcessX and Adobe Sign
By automating testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in ProcessX, you’re able to:
• ACCELERATE COMPLIANCE AND STREAMLINE VALIDATION WITH PROCESSX AND ADOBE ACROBAT SIGN
Automated Testing and Validation Workflows
Unify automated testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in a single platform. ProcessX, integrated with Adobe Acrobat Sign, empowers organizations to meet global regulatory requirements, reduce manual effort, eliminate process deviations, and ensure audit readiness with real-time dashboards and out-of-the-box compliance reporting.
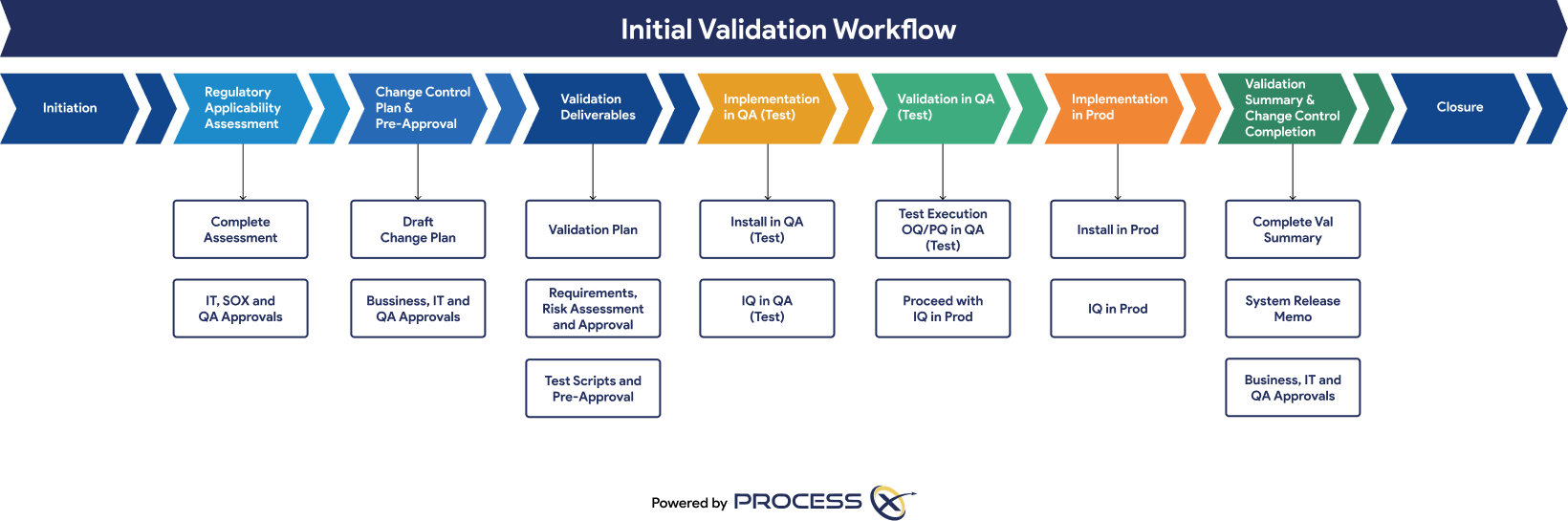
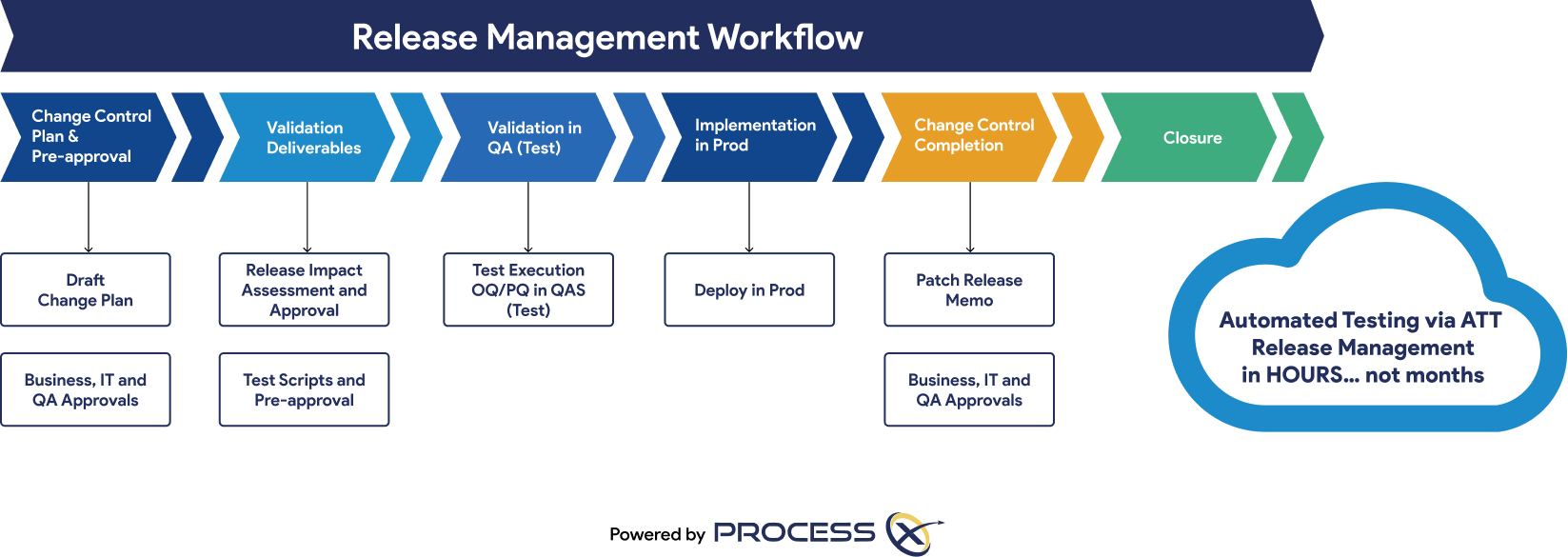
Regulatory Artifacts and On-Demand Reporting
ProcessX delivers out-of-the-box reporting templates and dashboards that save you time and give you peace of mind that you are audit-ready at a moment’s notice. Regulatory artifacts and reports include:
