GMP Manufacturing Workflows

Case Study
Digital Quality Process Prevents Erroneous Releases and Avoids High-Risk Deviations
Explore ways to achieve cost savings and practice cost avoidance. Adhere to Good Manufacturing Practice (GMP) standards with the help of ProcessX.
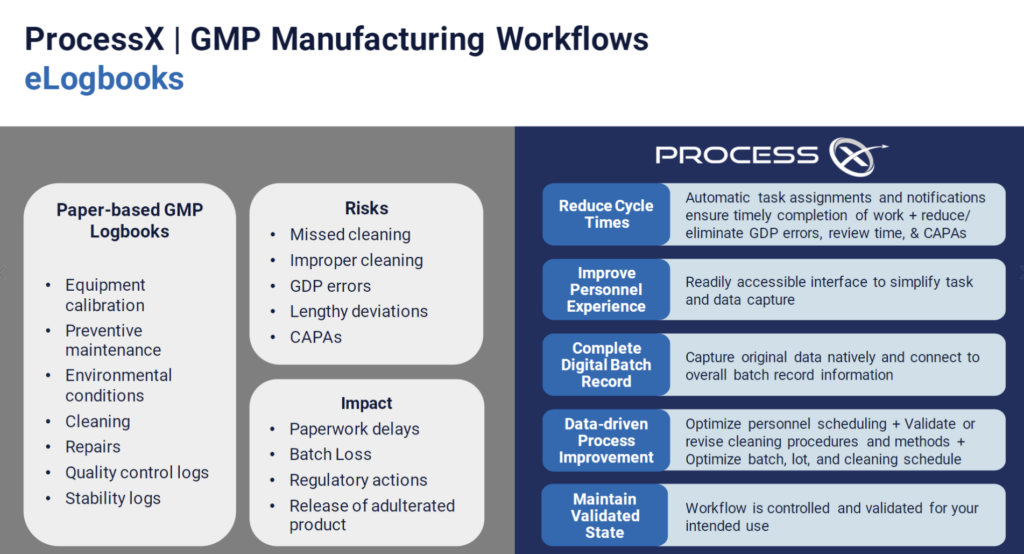
Manufacturing plants are still largely paper-based. Unfortunately, manual processes contribute to Good Documentation Practice (GDP) errors and lengthy deviations. And the expenses add up fast. Deviations range from $5,000 to $15,000 each, and corrective and preventive actions (CAPAs) range from $5,000 to $20,000 each. When it takes up to 90 days to resolve these issues, they really slow down your processes.
The potential cost savings with ProcessX is substantial. Besides decreasing the number of deviations, CAPAs, and GDP errors, ProcessX helps your life sciences organization:
- Deviation cost avoidance
- Mitigate re-runs and batch or lot losses
- Reduce the risk of recalls
- Shorten cycle times
- Decrease storage costs
- Increase audit response times
See how ProcessX saved 2,100 hours of manual work per year at 32 sites, which equates to 67,200 hours saved. By reducing high-risk deviations like erroneous releases, the multi-national pharmaceutical company improved its compliance and release cycle time.
Commit to Go Paperless
ProcessX GMP workflows digitally transform paper-based processes and records to help ensure your organization achieve and maintain compliance. They may even inspire your facility to be a factory of the future. After all, digital transformation and paperless validation systems are commendable goals in the life sciences industry. Advantages of paperless manufacturing include:
- Minimizing—or eliminating—hard-copy documentation
- Making documents available where and when they’re needed
- Improving the accuracy of documentation
- Tracking changes to documentation
- Ensuring regulatory compliance
GMP Manufacturing Workflows
ProcessX was designed to automate GxP processes and simplify regulated workflows and processes. It also helps to drive greater efficiency and improve product quality and patient safety. Use ProcessX to automate manufacturing workflows like:
- Logbooks
- Batch record review
- Paperless manufacturing
Benefits
ProcessX can automate any manual GxP process and deliver up to 5x speed and efficiency. Once you get started, you’ll be ready to:
- Build a variety of GxP processes quickly
- Use pre-configured forms as a starting point for departmental citizen developers
- Ensure that all workflows maintain a validated state and stay continuously compliant using the Cloud Assurance automation framework
Contact us today!
Fill out the form to contact us today about our manufacturing solutions with ProcessX
Manufacturing Use Case
Using manufacturing suite cleaning as an example, see how cleaning events are created and logs are kept in ProcessX.
Get in touch with our team to accelerate your digital transformation today.