Oracle Cloud Automated Testing Accelerated
Home Applications Oracle
• AUTOMATE ORACLE CLOUD COMPLIANCE AND REDUCE VALIDATION OVERHEAD BY UP TO 80%
Reduce Compliance Costs by up to 80%
Oracle Cloud applications are embedded with artificial intelligence (AI) and bring consistent processes to important business functions in enterprise resource planning, supply chain management, and human capital management. The applications help you improve internal and external engagements, increase your business’s agility, and react to change faster than ever before.
However, with four Oracle Supply Chain Management (Oracle SCM) releases a year, customers often struggle to manage all the compliance and validation activities required by global health authorities.
Oracle and USDM Life Sciences have partnered for two decades to provide world-class solutions that enable life sciences companies to meet FDA 21 CFR Part 11 regulatory requirements. USDM delivers trustworthy validation and release management that expertly manages GxP compliance for Oracle SCM customers through its Cloud Assurance service.
Add ProcessX to the Mix
USDM also developed capabilities for Application Lifecycle Management (ALM) and Validation Lifecycle Management (VLM) in ProcessX to automate activities and accelerate releases. Loaded with the Validation Verification Package for Oracle SCM, ProcessX gives you real-time access to all the necessary validation documentation and testing artifacts to seamlessly manage Oracle releases and demonstrate regulatory compliance.
USDM ensures that your compliance requirements are delivered correctly the first time and that your regulatory requirements are at the forefront of your technology implementation—they’re not an afterthought. We also help align IT and Quality stakeholders so you’re better able to consider all the requirements and processes needed to be audit-ready at a moment’s notice.
Automated ALM in ProcessX increases collaboration, compliance, and app deployment speed; results in greater efficiency; and eliminates fragmented systems. Automated VLM adds further functionality to support use cases like:
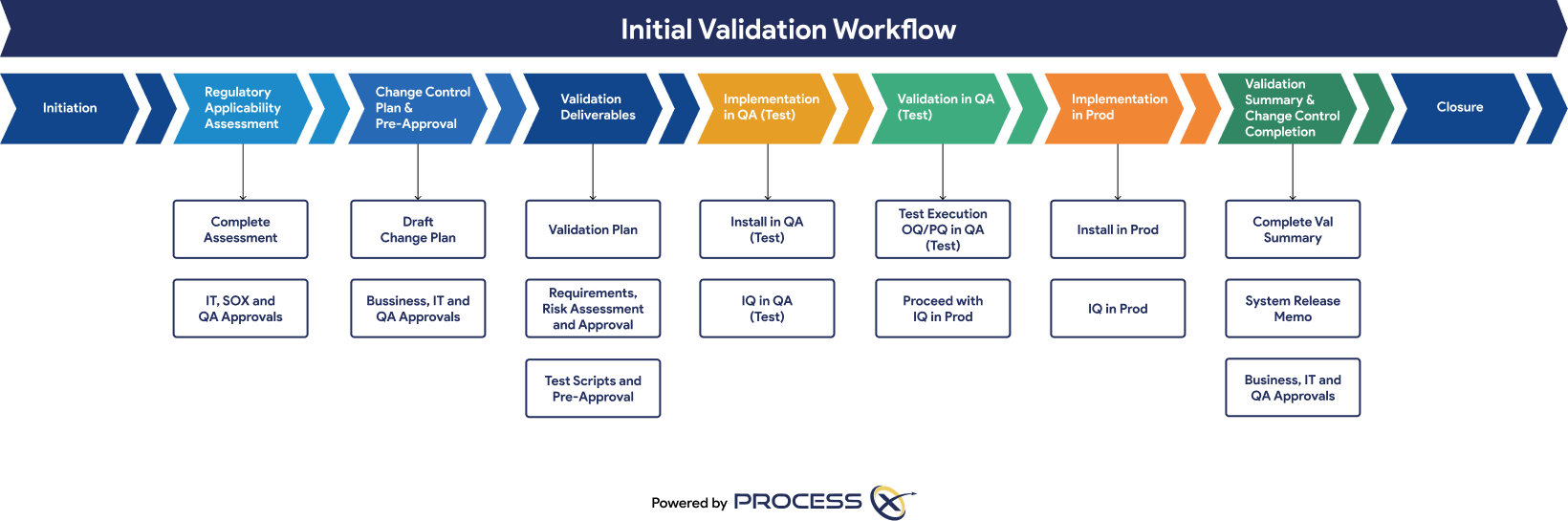
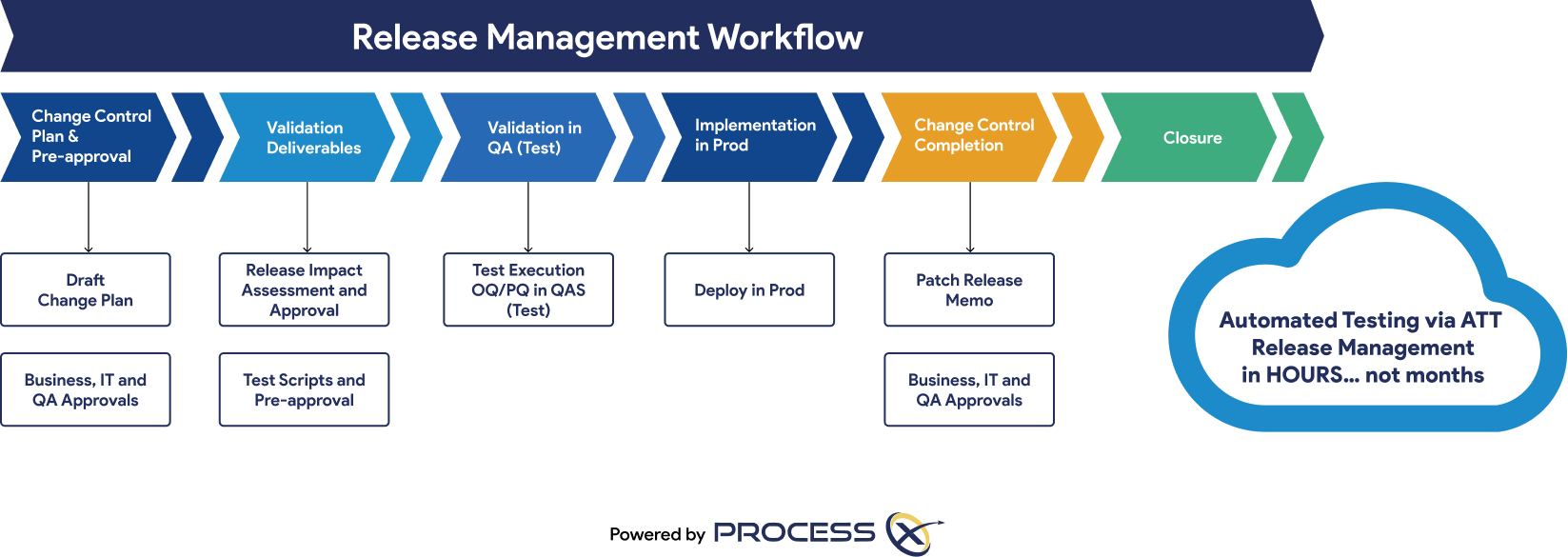
Oracle customers can onboard VLM and go live with Oracle SCM in six weeks. The test automation graphic shows the VLM process in ProcessX.
Compliance Solutions Delivered by USDM & ProcessX
Implementing USDM’s ServiceNow-based Risk and Cybersecurity solution delivers measurable business value:
• ACCELERATE COMPLIANCE AND STREAMLINE VALIDATION WITH PROCESSX AND ADOBE ACROBAT SIGN
Automated Testing and Validation Workflows
Unify automated testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in a single platform. ProcessX, integrated with Oracle Cloud, empowers organizations to meet global regulatory requirements, reduce manual effort, eliminate process deviations, and ensure audit readiness with real-time dashboards and out-of-the-box compliance reporting.
Regulatory Artifacts and On-Demand Reporting
ProcessX delivers out-of-the-box reporting templates and dashboards that save you time and give you peace of mind that you are audit-ready at a moment’s notice. Regulatory artifacts and reports include:
