Regulated ITSM for Life Sciences
Future-proof your regulated IT service management strategy with real-world case studies that show how life sciences organizations achieved continuous compliance, audit-readiness, and measurable operational efficiency. Read Regulated ITSM for Life Sciences to see how a proactive, digital quality approach transforms risk into resilience—and prepares your organization for what’s next.
Business Problem
Traditional ITSM platforms struggle to handle the rigor of GxP requirements. Change requests often require dual entry into both ServiceNow and Quality Management Systems (QMS), leading to inefficiencies, delays, and audit risks. Quality and IT teams are siloed, making collaboration on deviations, CAPAs, and change controls time-consuming and error-prone.
At the same time, validation processes slow release cycles and drain resources.
With the pace of digital transformation accelerating, organizations need a more intelligent, streamlined, and compliant solution.
The ProcessX Approach
USDM Life Sciences addresses these challenges through an AI-augmented, compliance-first strategy that connects IT and Quality using a unified ServiceNow platform.
IT Management Solutions for Life Sciences
Accelerate your IT service delivery with automated Software Development Lifecycle (SDLC), Agentic Artificial Intelligence (AI), and optimized impact assessment. USDM aligns IT Service Management (ITSM) with Configuration Management Database (CMDB) assessments and the Common Service Data Model (CSDM) for improved visibility and smarter release cycles—perfectly suited for regulated environments.
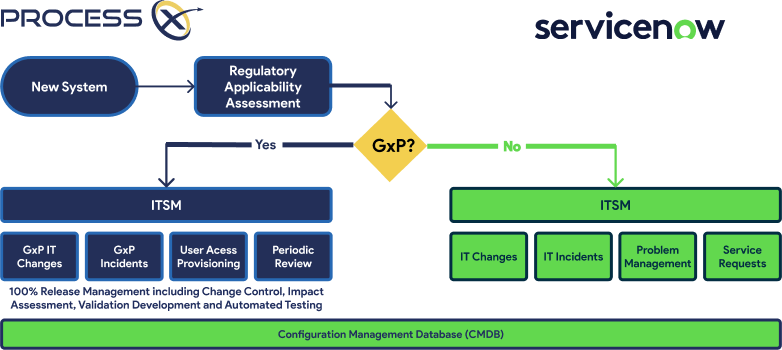
Key Capabilities
Unified Workflows Across IT and Quality using ProcessX and Agentic AI, USDM enables seamless, intelligent triage of service requests, incidents, and change controls. AI classifies the GxP relevance of each ticket and routes it appropriately—ensuring the right stakeholders are engaged and compliance actions are triggered automatically.
Segregated GxP and Non-GxP Flows ProcessX allows companies to maintain a single ServiceNow instance while keeping GxP workflows in a validated boundary. This segregation enables companies to innovate rapidly on non-GxP processes while maintaining tight control over regulated workflows.
CSA-Aligned Validation USDM pioneered CSA practices and builds automated validation directly into the ServiceNow workflow. Validation Lifecycle Management (VLM) in ProcessX simplifies system onboarding, change control, and system retirement—all with FDA-trusted audit trails.
CMDB & CSDM Strategy for Accuracy a well-structured CMDB and CSDM are critical for service visibility and impact analysis. USDM helps clients implement these frameworks specifically for GxP environments, boosting accuracy and enabling faster root-cause analysis.
Expected Business Outcomes
Partnering with USDM unlocks measurable improvements across IT and Quality teams:
50% faster approval of CAPAs and Change Requests thanks to intelligent routing and approval automation.
30% reduction in overdue deviations by centralizing data and embedding compliance workflows into ITSM.
100% traceability across lifecycle activities from validation to deployment with automated test and audit artifacts.
30% shorter release cycles through CSA-aligned validation, built-in automation, and reduced manual testing.
Why ProcessX?





We reduced our IT change approval cycle from 70 days to
just under 21—and we’re on track to reach sub-10 days with AI. The compliance and collaboration improvements have been enormous.
John Savage, Senior Director IT & R&D,
Life Sciences Systems of Record
This clip, from USDM’s 2026 Summit, industry leaders unpack how intelligent automation in regulated environments isn’t about replacing core systems—it’s about connecting them. By unifying structured and unstructured data across the GxP landscape, organizations gain AI-driven insights that surface risk early, enable continuous monitoring, and strengthen proactive compliance.
If you’re navigating digital quality, AI enablement, and cross-functional workflow optimization in a highly regulated environment, this conversation reframes the strategy entirely.
Our GxP IT System in Action
Curious how regulated ITSM actually works in a high-stakes, compliance-driven environment? See how one global life sciences company transformed fragmented IT service management into a unified, automated solution using ProcessX. In this real-world case study, the organization centralized change management, ticketing, validation, and asset tracking—all within a single GxP IT system. Designed specifically as one of the most effective IT management solutions for life sciences, ProcessX delivers the speed of modern automation while maintaining strict regulatory alignment. Read the Case Study: Transforming Regulated ITSM with ProcessX — A Scalable Compliance Engine
