Audit-Ready, Automated Testing for Salesforce
Home Applications Salesforce
• ENTERPRISE-GRADE E-SIGNATURES WITH COMPLIANCE AND AUTOMATION BUILT IN
Get Automated Testing, Validation Lifecycle Management, and Application Lifecycle Management in a Single App
As the top-ranked customer relationship management (CRM) provider, Salesforce offers unmatched configurability, scalability, and security for life sciences companies.
ProcessX and Salesforce
By automating testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in ProcessX, you’re able to:
• ACCELERATE COMPLIANCE AND STREAMLINE VALIDATION WITH PROCESSX AND SALESFORCE
Automated Testing and Validation Workflows
Unify automated testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in a single platform. ProcessX, integrated with SALESFORCE, empowers organizations to meet global regulatory requirements, reduce manual effort, eliminate process deviations, and ensure audit readiness with real-time dashboards and out-of-the-box compliance reporting.
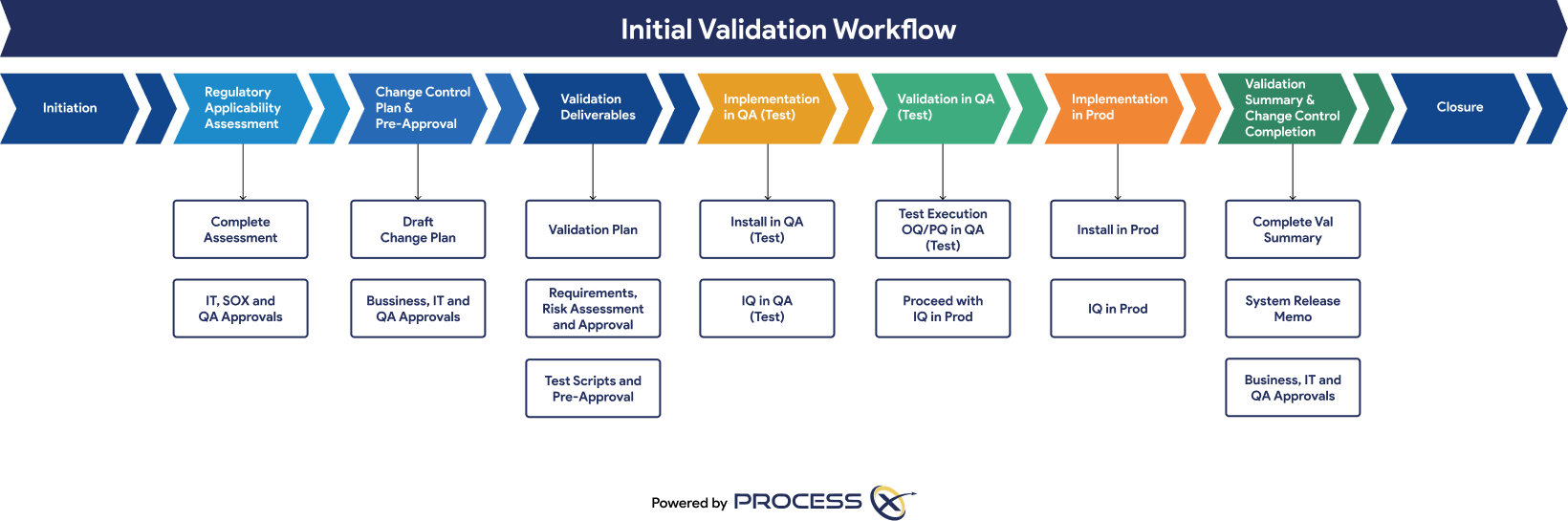
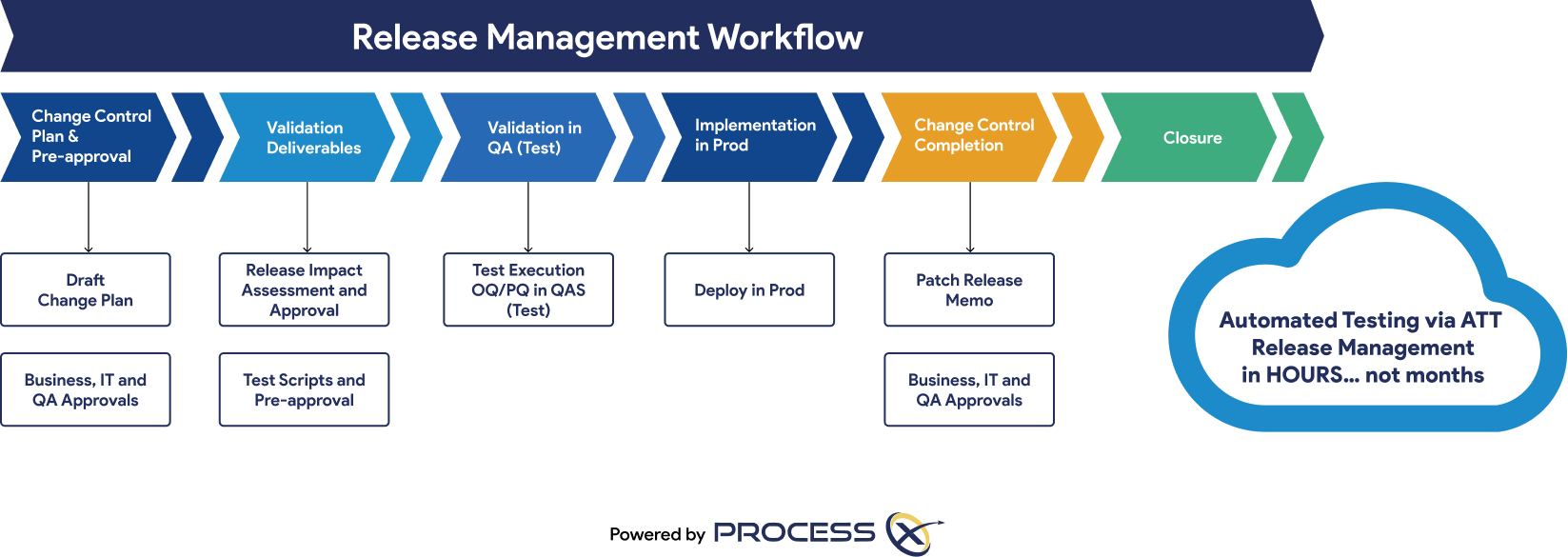
Regulatory Artifacts and On-Demand Reporting
ProcessX delivers out-of-the-box reporting templates and dashboards that save you time and give you peace of mind that you are audit-ready at a moment’s notice. Regulatory artifacts and reports include:
Regulated use cases for Salesforce include:
USDM streamlines your Salesforce implementation and ensures continuous compliance while managing mandatory updates and upgrading or expanding your existing system.
The longtime Salesforce and USDM partnership accelerates your organization’s digital transformation and helps extend Salesforce to regulated functions like clinical, laboratory, R&D, quality, manufacturing, and other GxP areas.
