Accelerate Paperless Validation with ProcessX

Achieve better lifecycle management in a superior, integrated Validation Lifecycle Management (VLM) and Application Lifecycle Management (ALM) platform.
ProcessX delivers VLM, ALM, automated regression testing and release management, and GxP services to keep you continuously compliant and ahead of the pack. Paperless validation is possible today. See it for yourself.

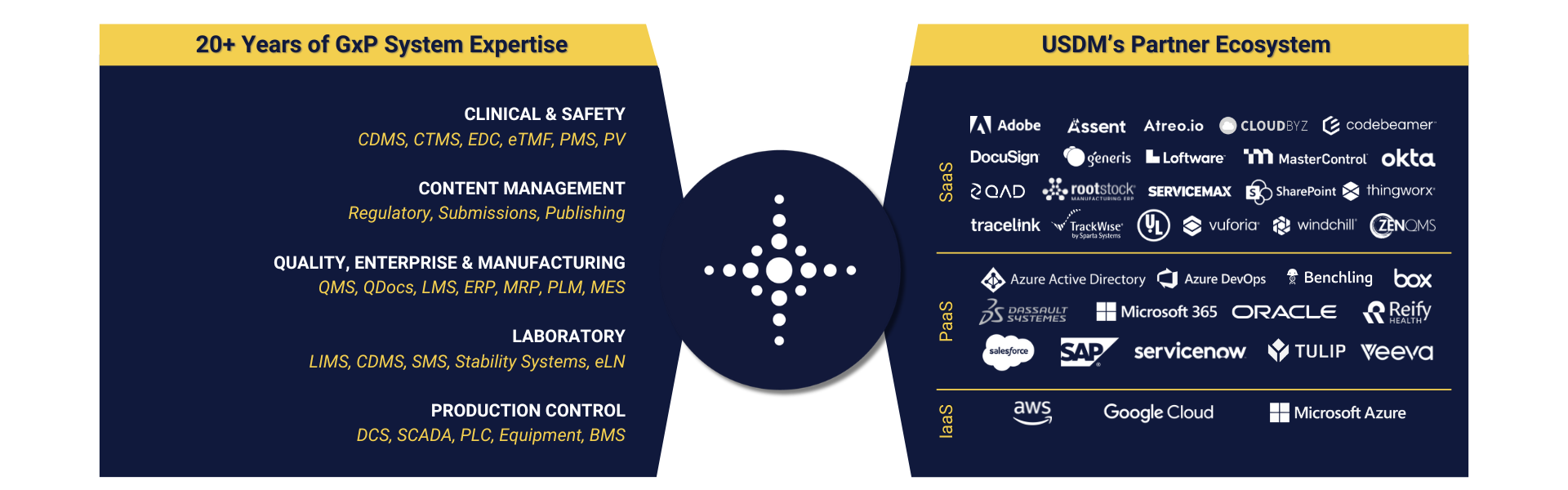
GxP and SOX Ready Capabilities
- Streamlined integration with your GxP or SOX SaaS systems
- Advanced, continuous compliance management
- Superior flexibility and customization for GxP or SOX workflows
- Enhanced user experience and citizen development
- Comprehensive GxP and SOX services and support
Business Outcomes You Can Bank On!

Reduce compliance costs by up to 80%

Harmonize Quality processes using a CSA methodology

Test Automation increases speed and efficiency by 5x

Manage all GxP, SOX and non-GxP systems using a single source of truth

Choose from dozens of audit-ready workflows
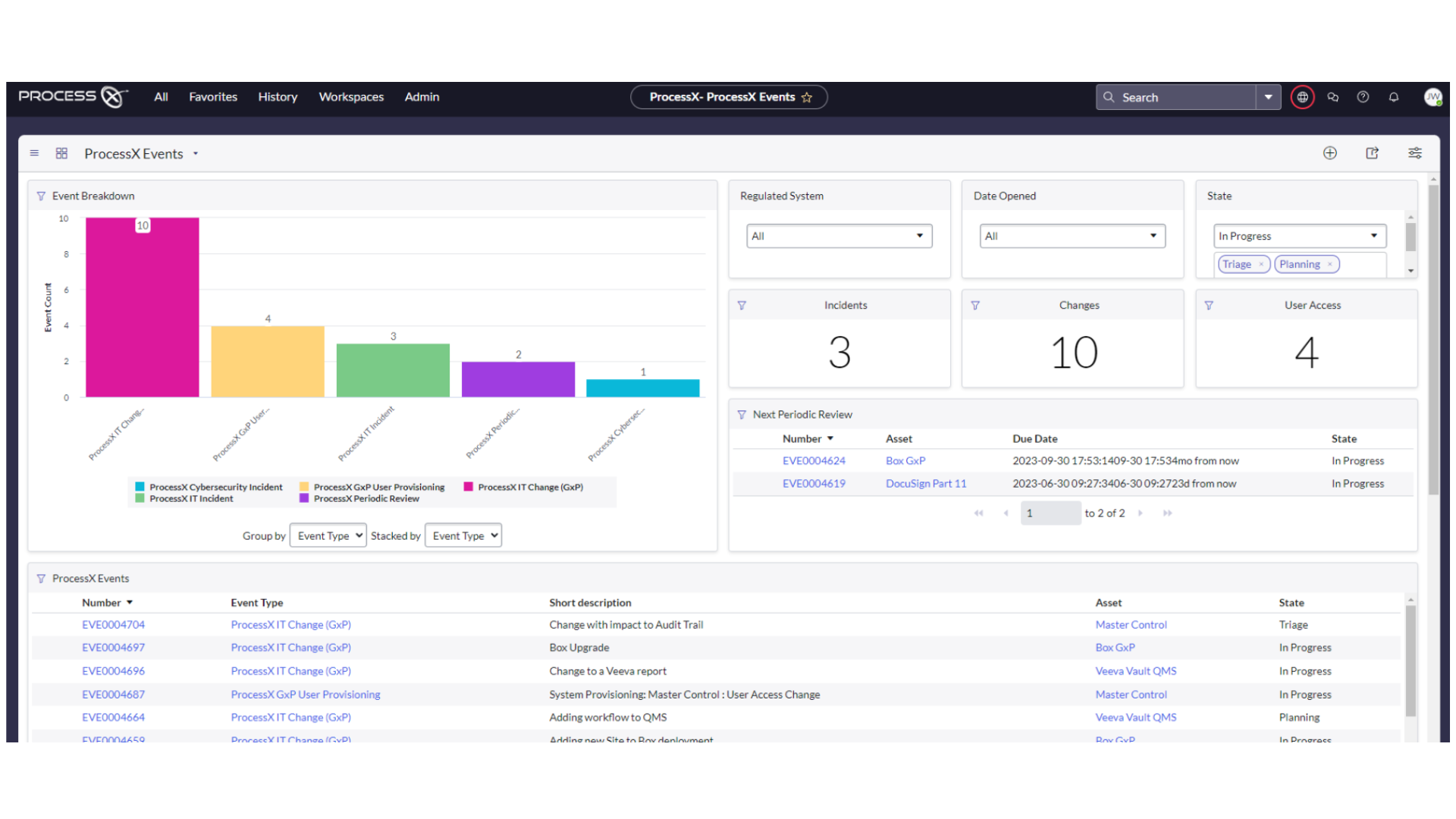
Deploy citizen development workflows from a low-code/no-code platform
Resources
Cloud Assurance as a Platform
Amplify the use and value of your GxP technology with automated and ongoing software testing.
Explore the digital-forward approach that will help your organization harmonize compliance and innovation and accelerate your journey from molecule to patient.
Read Now
WEBINAR
Managing GxP Workflows & Continuous Cloud Compliance with ProcessX and ServiceNow
Automate and scale your regulated business processes and simplify compliance and release management. Watch this on-demand webinar to learn how.
Watch Now
CASE STUDY
Clinical-Stage Oncology Company Automates Regulated IT Workflows with ProcessX
Learn how a clinical-stage oncology company automated regulated IT workflows for rapid transformation and increased operational efficiency.
Read Now
Get in touch with our team to accelerate your digital transformation today.