Cloud Assurance as a Platform

On-Demand Webinar
Managing GxP Workflows & Continuous Cloud Compliance with ProcessX and ServiceNow
Every day, life sciences companies must deal with events that have regulatory implications. Processes and data are often stored and managed in disparate systems and this fragmentation creates product delays and increases regulatory risk. Attempts at process automation often leave out real-time insight that would bring about transformative outcomes.
Imagine a platform that enables automation for new ways of working while simplifying, scaling, and maintaining regulatory compliance. ProcessX and Cloud Assurance as a Platform subscription is the unified solution you’re looking for.
Automated Testing and Release Management
Cloud Assurance as a Platform (CAaaP) is a cloud-based solution that automates the validation and testing of applications and software. It leverages USDM’s deep domain knowledge to validate, manage, and optimize GxP applications.
CAaaP seamlessly integrates with the validation lifecycle management (VLM) workflow in ProcessX. VLM can be used for initial implementation and enhancements, then a modified version is used for releases. It provides a single source of truth to manage everything associated with Application Lifecycle Management (ALM) and the implementation, upgrade, or enhancements of your GxP applications, including:
- Change Control
- Regulatory Applicability Assessments
- Risk Assessments
- Validation Deliverables and Artifacts
- GxP System Inventory
Because you’re moving from manual testing to automated testing, you’re able to streamline validation, simplify release management activities, and eliminate deviations. USDM uses starting point scripts to implement automated testing, which saves you money compared to the typical cost of entry.
This end-to-end process becomes your single source of truth for compliance.
It’s time to reimagine your business workflows and achieve transformative outcomes for your business units.
With USDM’s Cloud Assurance as a Platform and ProcessX solutions, you’re able to:
- Speed your time to market
- Maintain a perpetual state of audit readiness
- Lower your IT cost of ownership
- Enable AI and machine learning tools in your processes
- Harmonize your approach to digital quality
Unburden your teams of initial validation and ongoing compliance maintenance. CAaaP automated regression testing is hard at work ensuring that you remain continuously compliant.
Cloud Assurance cost savings range from 20% to 80%, depending on the extent of its integration and number of GxP systems. Get the validation cost comparison model to see how much you might save.
The FDA supports using new technologies and automation in the CSA methodology to help life sciences companies bring drugs and medical devices to market faster and more efficiently. To that end, CAaaP replaces subjective risk assessments and selective testing with cutting-edge technologies and delivery methods
To simplify release management and enable your organization to be consistent in performing tests, CAaaP automates testing activities and supports full-suite regression testing. Proprietary automation tools are configured to control the installation, configuration, management, and testing of cloud computing systems, which results in time savings and effective cloud management practices. Automated and ongoing software testing helps ensure that regulated systems remain in a state of compliance no matter how frequently vendors release updates, changes, and patches.
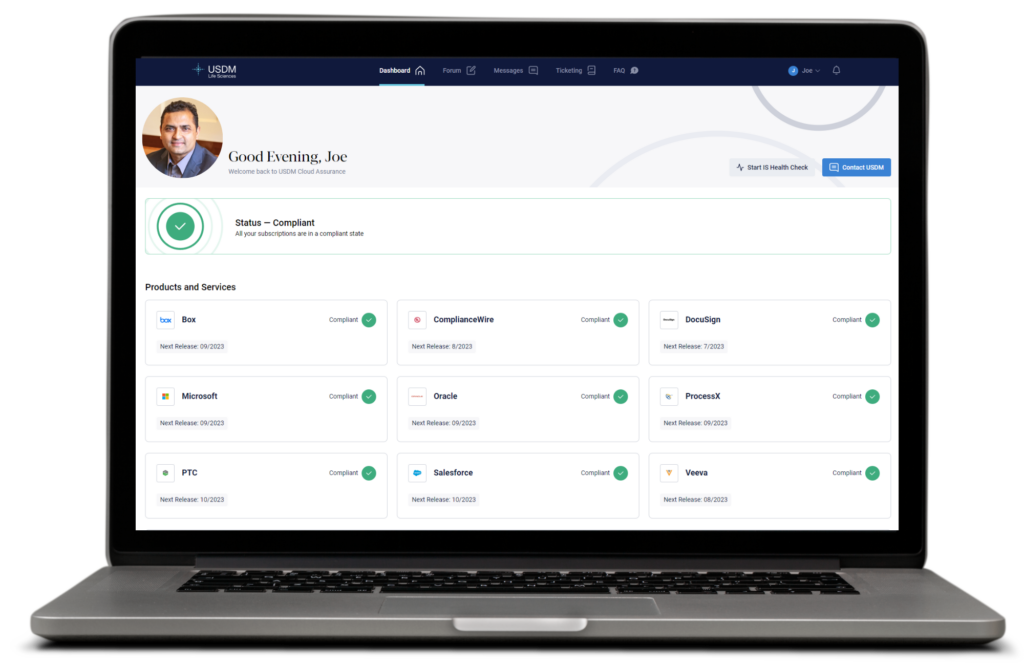
Measuring, monitoring, and managing the compliant state of your complex and ever-expanding GxP systems in the cloud requires maximum transparency and clear communication between you, your service partner, and technology vendors. Using the Cloud Assurance Digital Experience (Dx) web application, you get 24/7 access to a dashboard that tracks notifications and subscription activities. It’s also where you can download annual vendor audits and assurance reports. The Dx makes overseeing your GxP systems as easy as managing your bank account online.
Get in touch with our team to accelerate your digital transformation today.