Move faster with automated testing for SAP
Home Applications SAP
• ENTERPRISE-GRADE E-SIGNATURES WITH COMPLIANCE AND AUTOMATION BUILT IN
Get Automated Testing, Validation Lifecycle Management, and Application Lifecycle Management in a Single App
SAP helps companies of all sizes operate efficiently with solutions that centralize data management, help manage complex processes with ease, enable real-time access to information with the HANA In-Memory Database, and provide best-in-class solutions for Supply Chain & Manufacturing operations.
ProcessX and SAP
By automating testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in ProcessX, you’re able to:
• ACCELERATE COMPLIANCE AND STREAMLINE VALIDATION WITH PROCESSX AND SAP
Automated Testing and Validation Workflows
Unify automated testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in a single platform. ProcessX, integrated with SAP, empowers organizations to meet global regulatory requirements, reduce manual effort, eliminate process deviations, and ensure audit readiness with real-time dashboards and out-of-the-box compliance reporting.
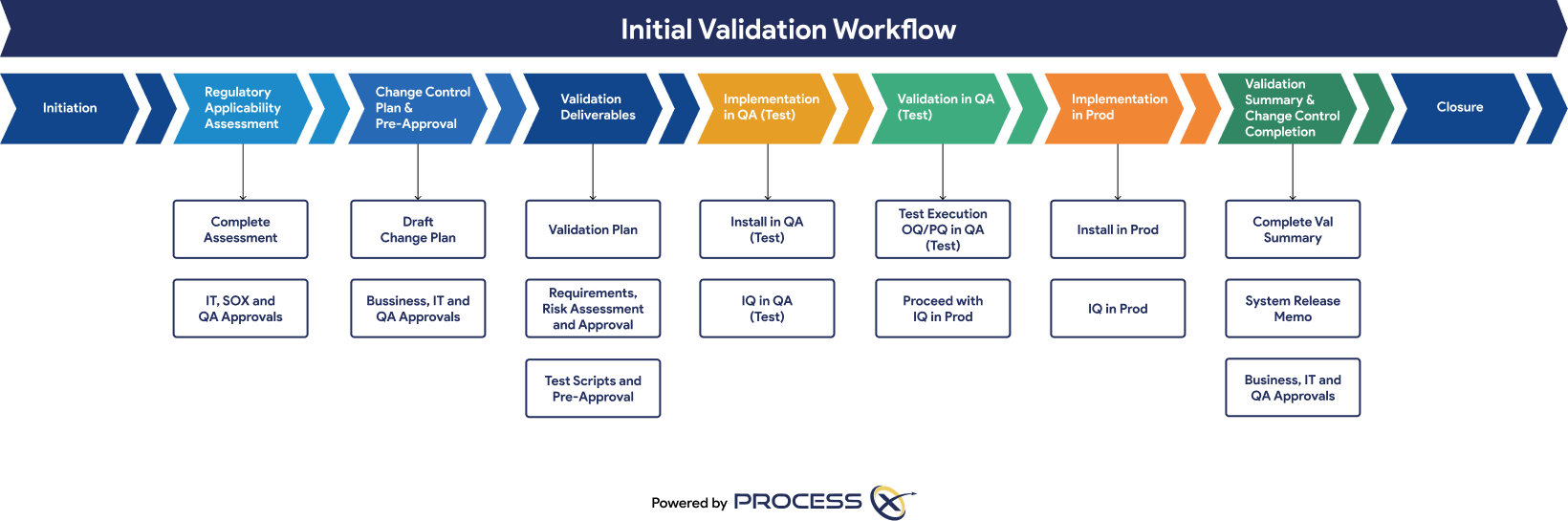
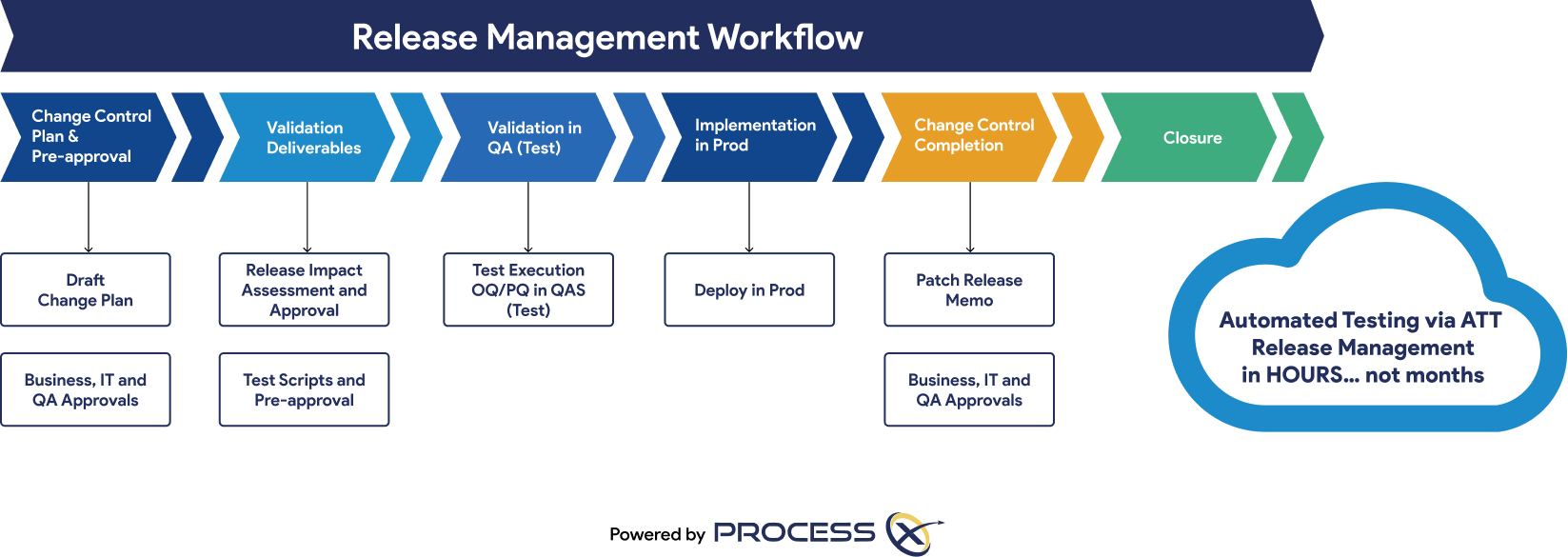
Regulatory Artifacts and On-Demand Reporting
ProcessX delivers out-of-the-box reporting templates and dashboards that save you time and give you peace of mind that you are audit-ready at a moment’s notice. Regulatory artifacts and reports include:
USDM enables life sciences organizations running SAP to meet U.S. Food and Drug Administration (FDA) 21 CFR Part 11 and other relevant regulatory requirements. Our trustworthy services create efficient processes and keep SAP fully compliant in the cloud at a fraction of the cost of traditional approaches.
