Minimize Your Veeva Compliance Burdens, Maximize Efficiency
Home Applications Veeva
• ENTERPRISE-GRADE E-SIGNATURES WITH COMPLIANCE AND AUTOMATION BUILT IN
Get Automated Testing, Validation Lifecycle Management, and Application Lifecycle Management in a Single App
Veeva Vault manages content and data in a single platform and enables life sciences organizations to deploy powerful applications that manage processes with related content, data, and workflows. USDM’s industry expertise ensures the program is expertly managed, implemented correctly, and adopted across the organization. ProcessX takes your Veeva Vaults even further.
ProcessX and Veeva
By automating testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in ProcessX, you’re able to:
• ACCELERATE COMPLIANCE AND STREAMLINE VALIDATION WITH PROCESSX AND VEEVA
Automated Testing and Validation Workflows
Unify automated testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in a single platform. ProcessX, integrated with Veeva, empowers organizations to meet global regulatory requirements, reduce manual effort, eliminate process deviations, and ensure audit readiness with real-time dashboards and out-of-the-box compliance reporting.
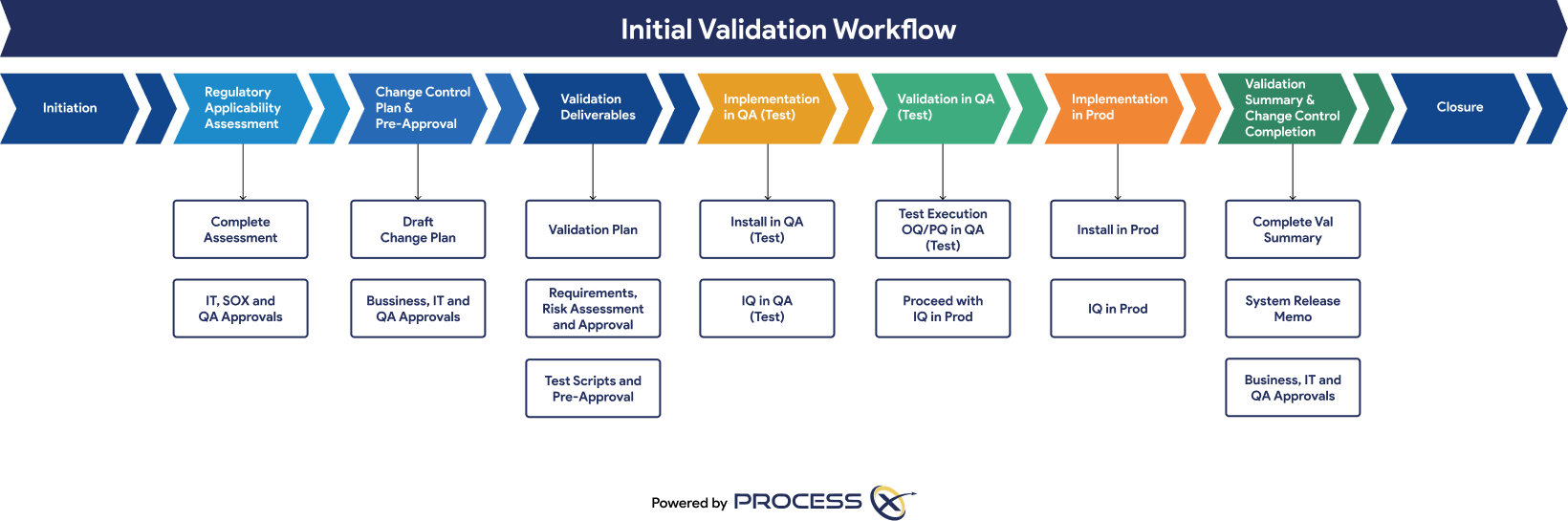
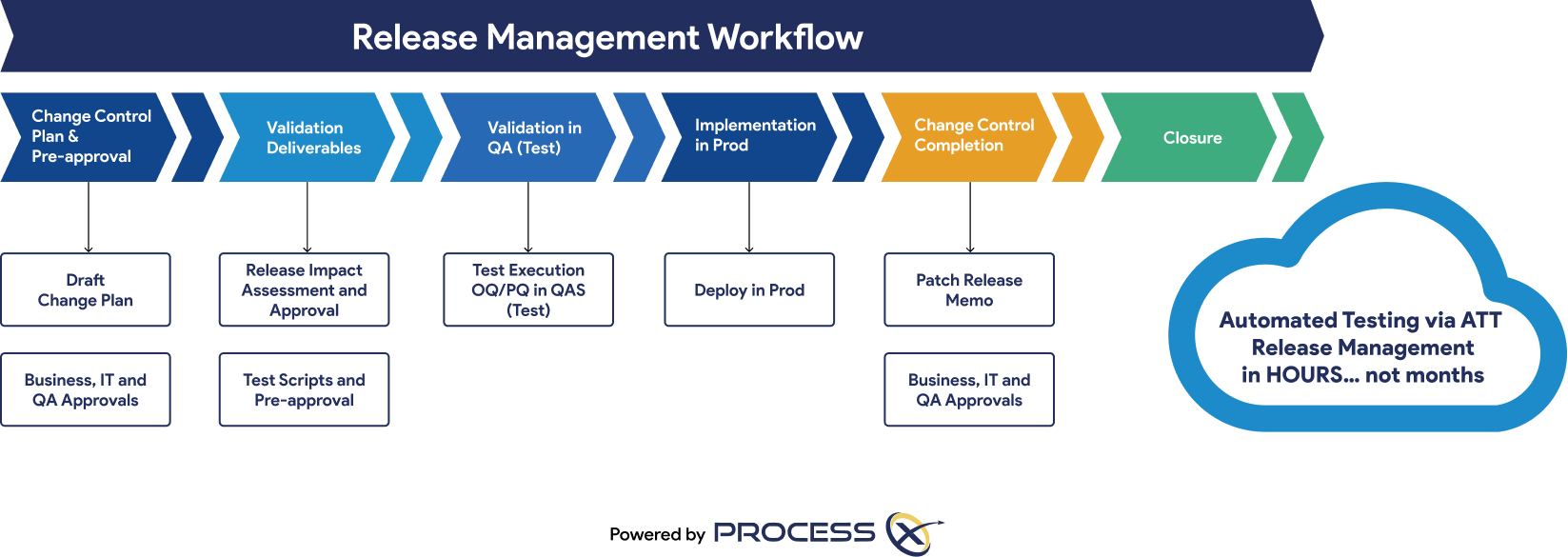
Regulatory Artifacts and On-Demand Reporting
ProcessX delivers out-of-the-box reporting templates and dashboards that save you time and give you peace of mind that you are audit-ready at a moment’s notice. Regulatory artifacts and reports include:
As a Premiere Services Partner of Veeva, USDM can also assist with expert program management, end-user training and communication, data migration, content governance, and post-go-live configuration and change management. For companies distributing medical devices, USDM’s global UDI services and Veeva’s UDI offerings assess your as-is state, provide regulatory and process guidance, and drive the transition to your to-be state.
