GMP, eForms, and eLogbooks in Life Sciences
Incorporating electronic forms and logbooks (eForms and eLogbooks) into automated workflows provide version control and audit trails, enable validation checks to minimize data-entry errors, and contribute to overall data integrity.
Designed to automate GxP processes and simplify regulated workflows, ProcessX drives greater efficiency in processes that help boost product quality and ensure patient safety. Explore ways that eForms and eLogbooks in ProcessX contribute to cost savings and prepare your organization to practice cost avoidance.
eForms Streamline the Data Capture Process
Paper-based forms present many challenges. Are they filled in completely? Can you read the person’s handwriting? Do the form fields match your database fields? Are the forms filed properly and easily accessible?
You get the idea.
To facilitate accurate and efficient data capture, ProcessX lets you create with ease the forms you need. Integrating these forms into automated workflows helps your organization manage manufacturing and quality control processes with up-to-date information. To further enhance accuracy, your forms can include validation checks to ensure that input meets predefined criteria.
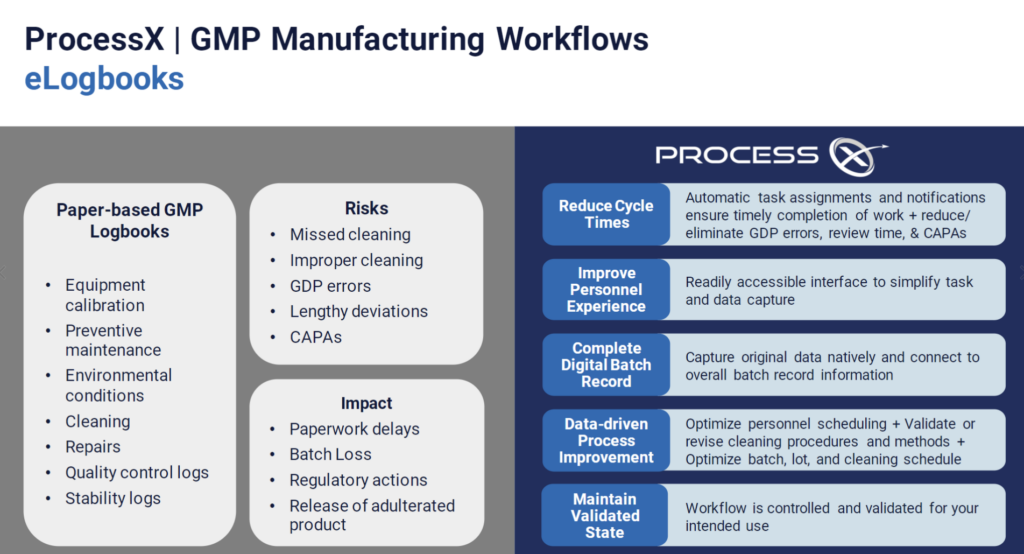
eLogbooks Keep Information Organized and Accessible
When using paper-based logbooks for activities like equipment calibration, cleaning, and preventive maintenance, your company is at risk of missed tasks, Good Documentation Practice (GDP) errors, and lengthy deviations.
ProcessX empowers your team leaders to automate paper-based processes and records, meet regulatory requirements, and maintain continuous compliance.
Automated task assignments and notifications keep employees informed of their responsibilities. An easy-to-use interface simplifies data capture. Data-driven process improvement helps to optimize scheduling.
ProcessX Saves You Time and Money
Manufacturing plants are still largely paper-based and their manual processes often lead to GDP errors and deviations. Expenses like that add up fast.
Deviations range from $5,000 to $15,000 each, and corrective and preventive actions (CAPAs) range from $5,000 to $20,000 each. When it takes up to 90 days to resolve these issues, they really slow down your processes.
The potential cost savings with ProcessX are substantial. Besides decreasing the number of deviations, CAPAs, and GDP errors, ProcessX helps your life sciences organization:
- Avoid the cost of deviations
- Mitigate re-runs and batch or lot losses
- Reduce the risk of recalls
- Shorten cycle times
- Decrease storage costs
- Increase audit response times
ProcessX can automate any manual GxP process and deliver up to 5x speed and efficiency. Once you get started, you’ll be ready to:
- Build a variety of GxP processes quickly
- Use pre-configured forms as a starting point for departmental citizen developers
- Ensure that all workflows maintain a validated state and stay continuously compliant using the USDM Cloud Assurance automation framework
Use ProcessX to automate manufacturing workflows like eLogbooks, eForms, and batch record reviews in your organization. Contact us to get started today.
Contact us today!
Fill out the form to contact us about our eForms, eLogbooks and other solutions!
Get in touch with our team to accelerate your digital transformation today.

