Audit-Ready, Automated Testing for Box
Home Applications Box
• ENTERPRISE-GRADE E-SIGNATURES WITH COMPLIANCE AND AUTOMATION BUILT IN
Get Automated Testing, Validation Lifecycle Management, and Application Lifecycle Management in a Single App
Box GxP supports a variety of life sciences use cases, including contract research organization (CRO) and contract manufacturing organization (CMO) collaboration, clinical collaboration, clinical dataset management, Quality document management, mergers and acquisitions (M&As), and post-merger integration (PMI).
ProcessX & Box
By automating testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in ProcessX, you’re able to:
• ACCELERATE COMPLIANCE AND STREAMLINE VALIDATION WITH PROCESSX AND BOX
Automated Testing and Validation Workflows
Unify automated testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in a single platform. ProcessX, integrated with Box, empowers organizations to meet global regulatory requirements, reduce manual effort, eliminate process deviations, and ensure audit readiness with real-time dashboards and out-of-the-box compliance reporting.
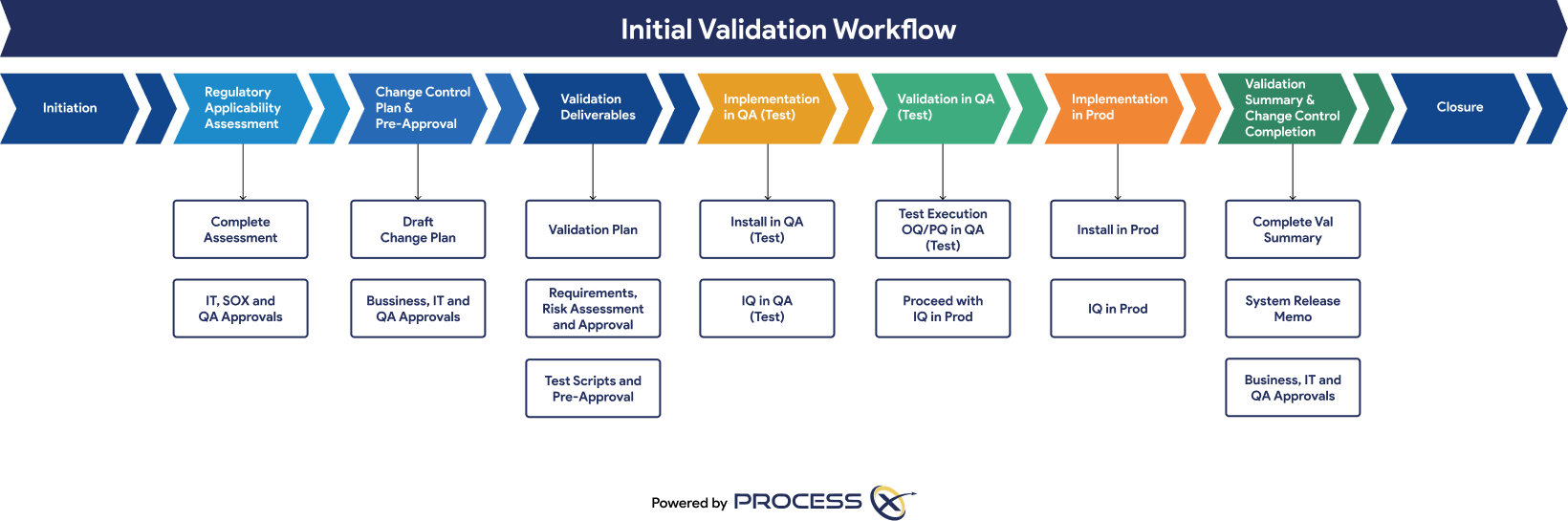
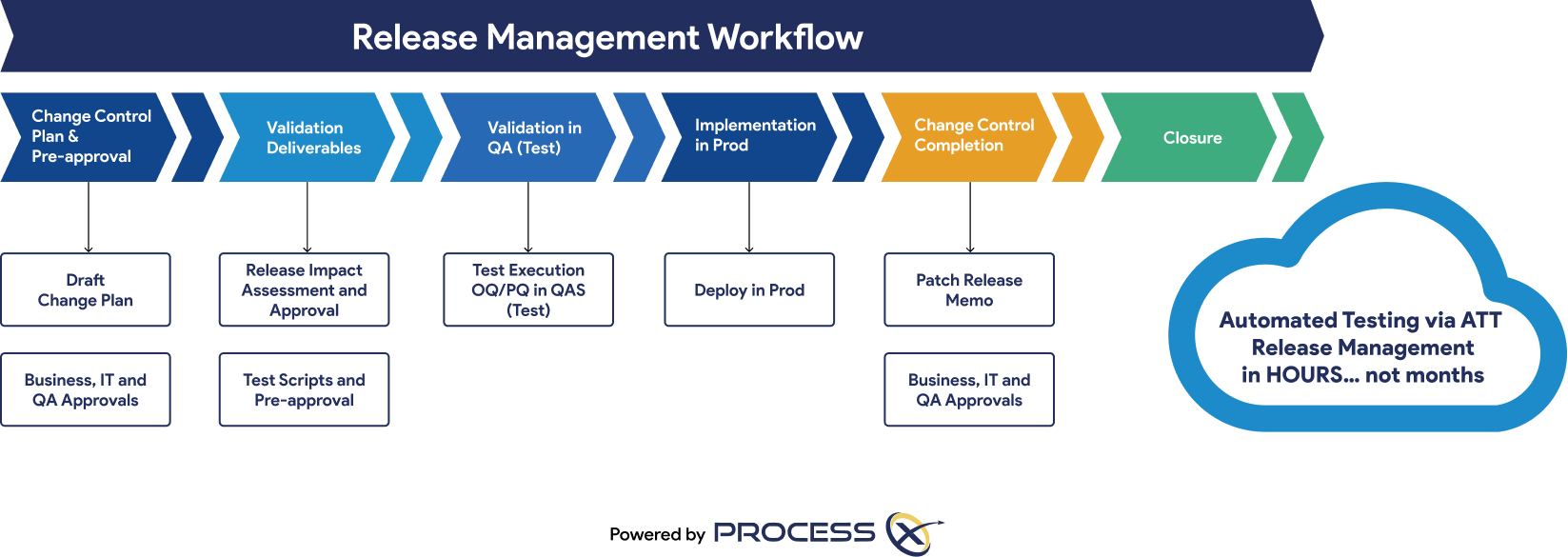
Regulatory Artifacts and On-Demand Reporting
ProcessX delivers out-of-the-box reporting templates and dashboards that save you time and give you peace of mind that you are audit-ready at a moment’s notice. Regulatory artifacts and reports include:
Why Automation Scales (or Stalls)
This clip, from USDM’s 2026 Summit, explores how scalable automation starts with strong metadata foundations. Without structured, metadata-driven processes in place, organizations can’t effectively scale automation—at least not without adding significant human effort.
To reach the next level of intelligent, enterprise-wide automation, getting metadata right from the start is essential.
