DocuSign Automated Testing Accelerated
Home Applications DocuSign
• ENTERPRISE-GRADE E-SIGNATURES WITH COMPLIANCE AND AUTOMATION BUILT IN
Get Automated Testing, Validation Lifecycle Management, and Application Lifecycle Management in a Single App
DocuSign helps life sciences organizations automate the preparation, signing, and management of agreements. It enables U.S. Food and Drug Administration (FDA) 21 CFR Part 11 and Health Insurance Portability and Accountability Act (HIPAA) compliant eSignatures. It’s the world’s first choice for electronic signatures on practically any device.
ProcessX & DocuSign
By automating testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in ProcessX, you’re able to:
• ACCELERATE COMPLIANCE AND STREAMLINE VALIDATION WITH PROCESSX AND BOX
Automated Testing and Validation Workflows
Unify automated testing, Validation Lifecycle Management (VLM), and Application Lifecycle Management (ALM) in a single platform. ProcessX, integrated with Box, empowers organizations to meet global regulatory requirements, reduce manual effort, eliminate process deviations, and ensure audit readiness with real-time dashboards and out-of-the-box compliance reporting.
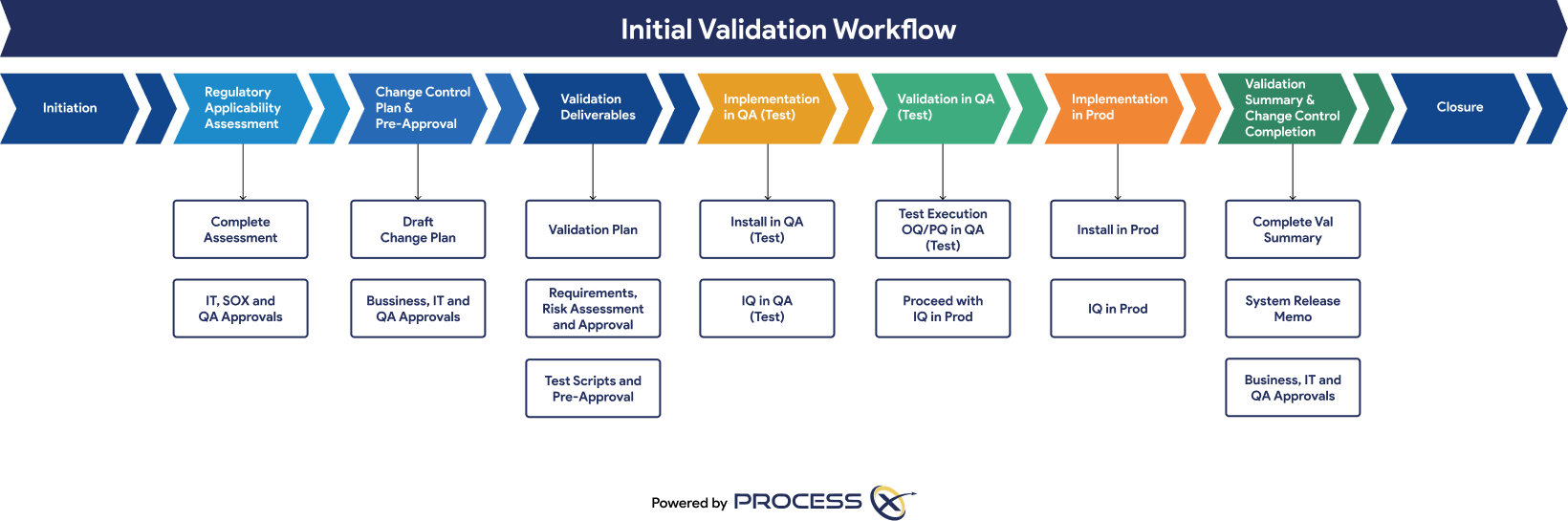
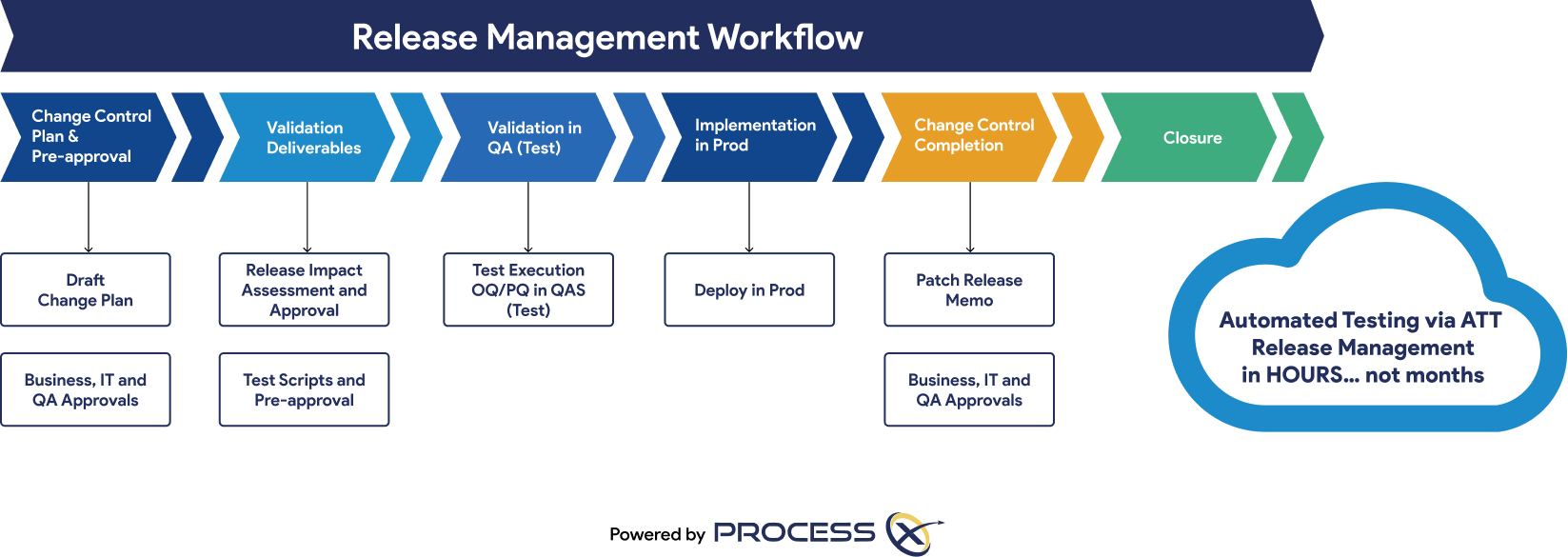
Regulatory Artifacts and On-Demand Reporting
ProcessX delivers out-of-the-box reporting templates and dashboards that save you time and give you peace of mind that you are audit-ready at a moment’s notice. Regulatory artifacts and reports include:
The DocuSign and USDM partnership has resulted in deployments at the Top 20 global biopharma and medical device companies and in high-growth life sciences organizations.
USDM has perfected DocuSign implementation to get you up and running in as fast as seven days. There’s no need to create your documents from scratch; we’ve optimized more than 175 DocuSign systems using our methodologies, document templates, and processes. From there, system configurations can be modified using change control processes.
Streamline and optimize your GxP workflows and DocuSign electronic signatures with ProcessX and reduce your compliance costs by up to 80%!
