How modern architecture is unlocking faster decisions and scalable compliance across life sciences IT ecosystems
The life sciences sector has no shortage of intelligent tools or compliance platforms. Yet many organizations still find themselves encumbered by inefficiencies—lost time, redundant approvals, and risk-prone handoffs. It’s not due to a lack of innovation—it’s due to the way systems are designed to work together.
In 2026, operational resilience is not just about automation. It’s about smart architecture.
While many regulated organizations have invested heavily in platforms like ServiceNow, Jira, or QMS tools, their return on investment often stalls. Why? Because critical teams—IT, Quality, and Compliance—are operating on disconnected systems, often duplicating effort and introducing friction where speed is essential.
Let’s be clear: this isn’t a tooling problem. It’s a workflow design challenge.
What’s Slowing Down IT-Quality Collaboration?
In highly regulated environments, IT Operations and Quality teams must stay tightly aligned—especially when managing changes that impact GxP systems. But too often, these teams operate in siloed systems, with compliance data and infrastructure insights split across platforms.
This architecture leads to three critical bottlenecks:
- Manual data reconciliation between ServiceNow and legacy QMS tools
- Inefficient, multi-step approvals that delay execution
- Redundant meetings and documentation to satisfy compliance without optimizing performance
The result? Slower decision-making, limited visibility, and increased operational risk—especially as life sciences organizations ramp up AI and cloud adoption.
The Cost of Fragmented Workflow Architecture
For a mid-sized biotech or pharmaceutical company, misaligned workflows aren’t just frustrating—they’re financially significant. On average, teams lose hundreds of hours annually to duplicate data entry, rework, and fragmented change management processes.
Here’s what’s typically at stake:
- Time-to-decision delays: Swivel-chair approvals between platforms
- Operational risk exposure: Limited traceability across GxP assets
- Hidden costs: Manual validation cycles and redundant analysis efforts
When systems aren’t natively connected, human intervention becomes the glue—and that’s expensive, error-prone, and unsustainable at scale.
Design for Compliance and Speed—Not One or the Other
Regulated industries have traditionally taken a compliance-first approach: design workflows to pass audits, even if it sacrifices speed and collaboration. But forward-thinking organizations are turning that model on its head.
Instead of customizing general-purpose IT platforms beyond recognition, they’re asking:
What if compliance could live where the data already exists?
With fit-for-purpose solutions like ProcessX, teams are redesigning workflows to live directly within platforms like ServiceNow, enabling GxP-ready automation without costly custom builds or revalidation cycles.
By embedding compliance controls into native IT operations:
- Data duplication disappears
- Change visibility improves across Quality and IT
- Teams reclaim time and focus on high-impact decisions
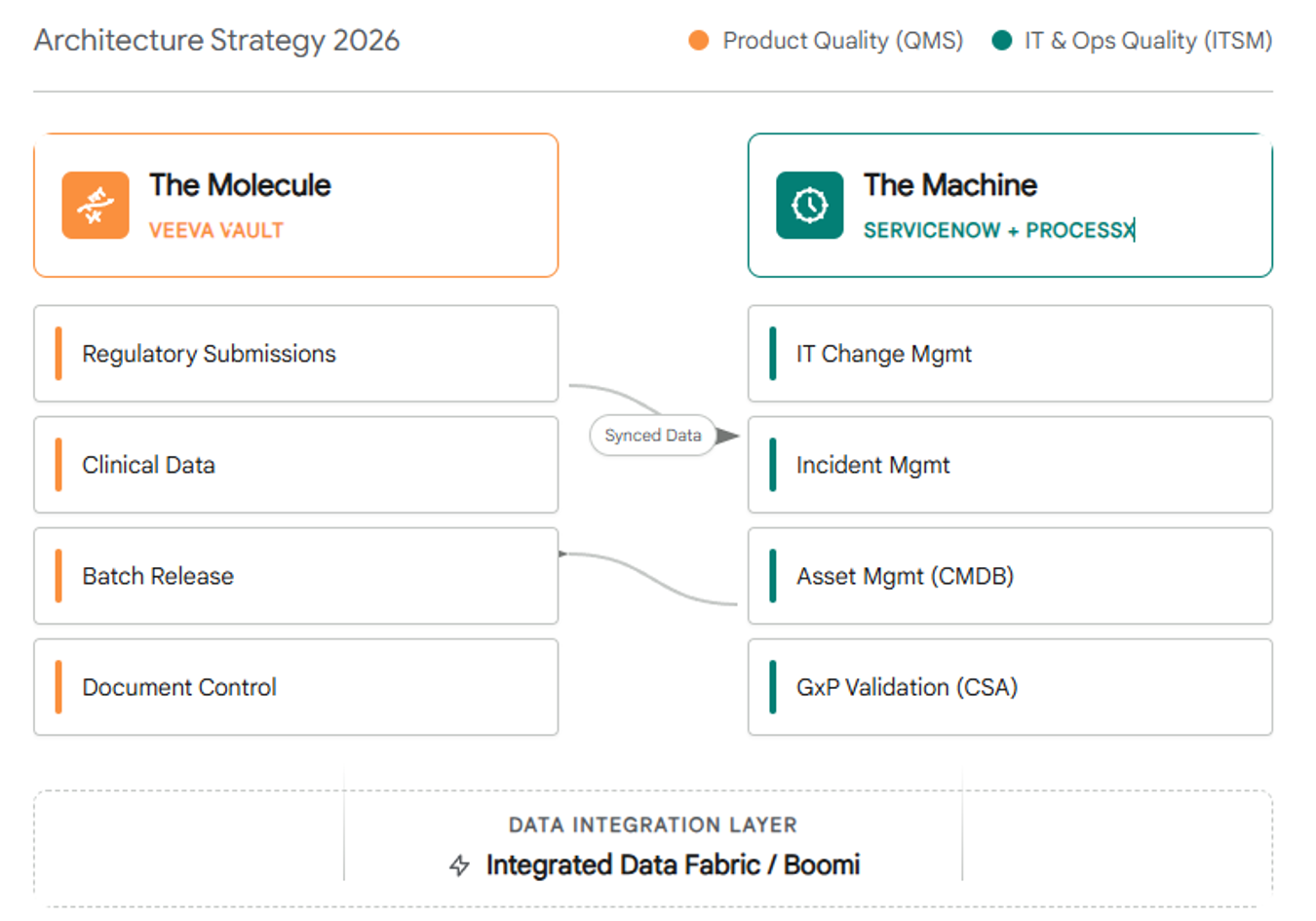
The Smarter Architecture Model
Rather than forcing all workflows into one monolithic system, the smarter approach is to assign platforms to their strengths:
| Platform | Purpose |
| QMS | Product Quality (Clinical, Submissions) |
| ServiceNow + ProcessX | Operational Quality (IT, Infrastructure, AI Agents) |
With this split-system model, life sciences companies gain the agility to scale AI and automation, while maintaining audit-readiness and data integrity across the enterprise.
Real Results from Better Workflow Design
In organizations that have adopted modern workflow architecture, we’ve seen:
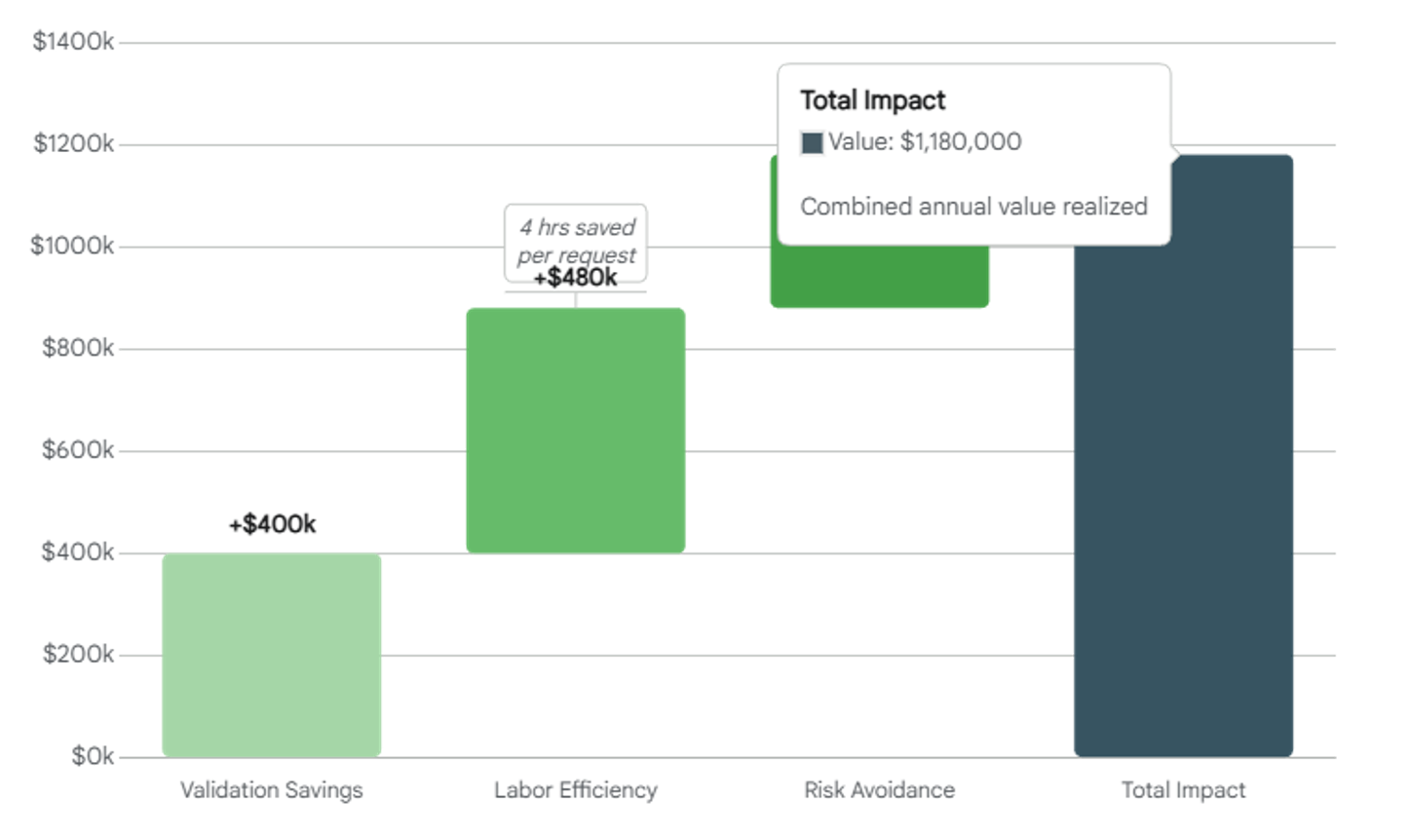
- 80% reduction in change management cycle time
- Unified decision-making across Quality and IT
- Elimination of $1M+ in annual waste due to manual compliance work
And critically, a shift from reactive compliance to proactive risk management—empowering teams to make better decisions, faster.
Reimagine Workflow with ProcessX
At ProcessX, we’re building more than automation—we’re building confidence in compliance at scale.
Whether you’re navigating GxP system upgrades, AI enablement, or global platform integration, our solutions are designed to:
- Reduce manual overhead and error-prone processes
- Support always-on audit-readiness
- Accelerate enterprise-wide change execution
You don’t need to rip and replace your systems to unlock this value—you just need to rethink how they work together.
Explore the Strategy in Action at the USDM Summit
The challenges and opportunities discussed above aren’t hypothetical—they’re real-world issues USDM encounters every day across the life sciences landscape.
At the USDM Life Sciences Summit, industry leaders dive deeper into these topics with firsthand insights, tangible use cases, and actionable strategies tailored for CIOs, Heads of Quality, and CFOs preparing for the future of regulated operations.
Watch USDM Summit 2026 On-Demand to learn more about this topic.